India’s Biological E to begin Phase III trial, production from August.
Byline By- Balkrishna
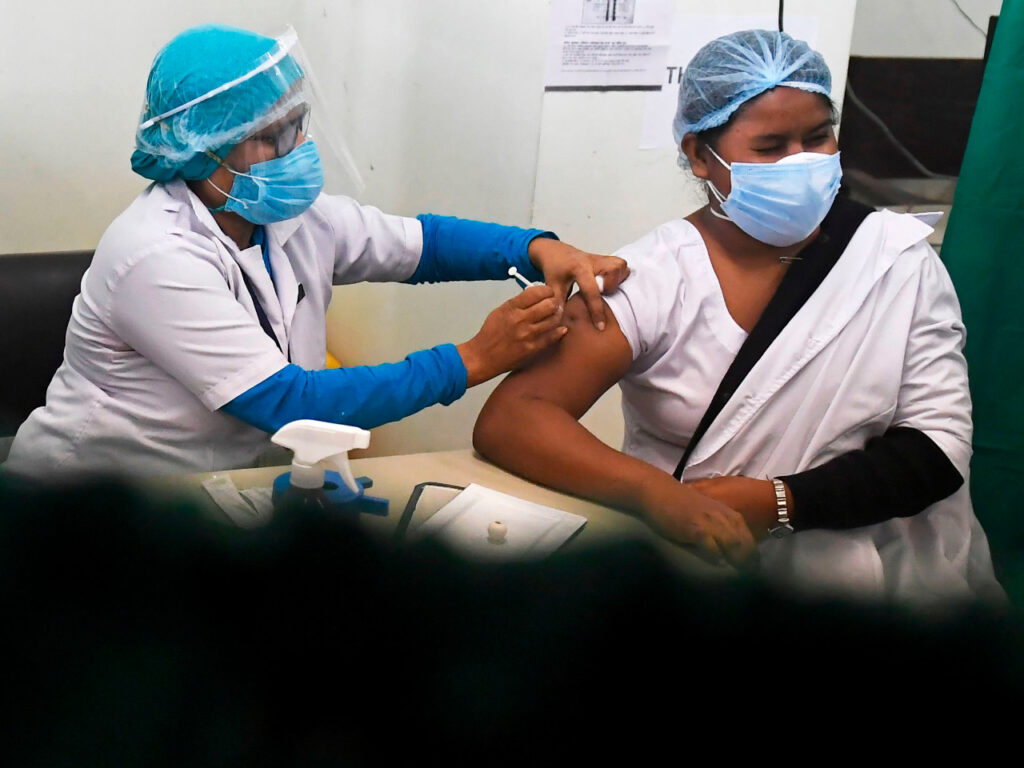
India’s Biological E. Ltd will be beginning Phase III trial of its COVID-19 vaccine and plans to create 75 million to 80 million portions per month from August.
The organization has built up the vaccine with Baylor College of Medicine in Houston and Dynavax Technologies Corp. Toward the end of last month, it got an endorsement from India’s medication controller to lead a Phase III clinical trial, which Managing Director Mahima Datla said would start soon.
Government authorities have said the vaccine, which utilizes the recombinant-protein tech wherein aharmlessagent is utilized to stimulate an immune reaction in cells, could be carried out in the country from August.
Datla said Biological E. would apply for crisis use authorization (EUA) for the medication dependent on government guidance.
Datla declined to comment on any firm arrangement of contract-produce with the Johnson and Johnson vaccine. She further added that Biological E. was hoping to make around 600 million portions of the J&J vaccine every year.
J&J said a month ago it was in talk with India’s administration to start a clinical preliminary of its single-portion shot.
India, engaging the world’s most exceedingly terrible leap in Covid cases, has somewhat or completely vaccinated only about 10% of its 1.35 billion individuals. It has controlled a total of 163 million doses of the AstraZeneca shot and a locally made one called Covaxin.
The nation has also got portions of the Sputnik V immunization from Russia however it has not been dispatched at this point in the country. India has additionally asked Pfizer/BioNTech and Moderna to offer their shots to the country.
